- Technologies
- Furnace atmosphere
The "furnace atmosphere" means a gas to be filled and heated in the furnace by which the
product (workpiece) is indirectly heat-treated. The atmosphere gases include air,
inert gases, hydrogen (reducing gas), and others, which are heated up to 1000 - 2500℃
depending on the properties of the workpiece and purpose of the heat treatment.
What are the atmosphere gases?
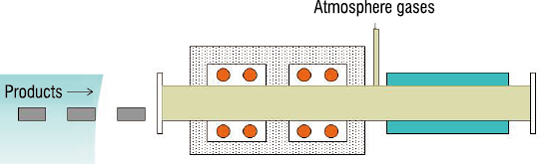
●A gas to be put into the furnace
●How to fill the furnace with an atmosphere gas
What kind of gas to be used?
From where to put in the gas?
How much gas to put in?
How to provide the gas?
Why is it necessary to use an atmosphere gas?
−Protection against oxidation
■Heating in air causes the workpiece to react with oxygen which negatively affects the product's appearance
and properties.
−Protection against decarburization
■While a carbon-added iron shows excellent properties, they will return to ordinary irons once they lose
the carbon from their surfaces (decarburization).
●Use of atmosphere gases to improve product quality
−Carburization
■Conversely to the aforementioned decarburization, an addition of carbon to the surface (carburization)
improves properties of the product.
−Nitrization
■Provision of nitrogen to the surface of steel is called nitrization which improves surface properties of the
product.
Type of atmosphere gases
●Frequently used atmosphere gases
−Exothermic generation gas
■DX gas
−Endothermic generation gas
■RX gas
−Inert gases
■Nitrogen, Argon
−Hydrogen gas
■Hydrogen gas
−Ammonia cracking gas (AX gas)
■Mixture of nitrogen and hydrogen obtained by dissociating ammonia
−Methanol cracking gas
■Obtained by dissociating Methanol
Exothermic generation gas
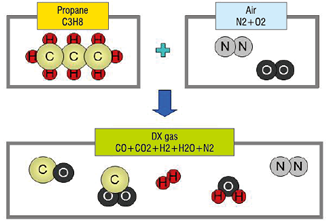
●This gas is called a DX gas
●Produced from Propane or Butane
Endothermic generation gas
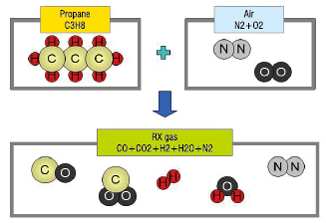
●This gas is called an RX gas
●Produced from Propane or Butane
Features of Generated gases
●Generated gasses are basically combustible mixtures of CO, CO2, H2, H2O, and N2.
●Generated gas composition is determined by the mixing rate of the material gas and air.
●Capable of reducing oxides of iron, steel, and copper, depending on the mixing rate and temperature.
Inert gases
●It does not react with the product, as it name suggests.
(While nitrogen may nitride some metals, it is generally considered inert.)
●Neither oxidation nor reduction takes place on the product.
(Trace amount of oxygen contained in commercially available inert gasses could slightly oxidize the product.)
●Nitrogen is lighter than air, and argon is heavier than air.
Hydrogen gas
●Reduces many kinds of metal oxides.
−Example:
FeO+H2→Fe+H2O
●Mishandling of hydrogen may cause explosion accident.
●Much lighter than air.
Cracked (generation) gasses
−Produced by heat dissociation of ammonia in a designated generating furnace.
Composed of hydrogen and nitrogen.
Combustible and explosive due to hydrogen content.
Efficient source of large amount of hydrogen, without needs of hydrogen tubes.
Usable for sintering or brazing of iron based materials.
●Methanol cracked gas
−Produced by heat dissociation of methanol in a designated generating furnace.
Composed mainly of CO and hydrogen.
Capable of carburizing steels such as bearing steel.
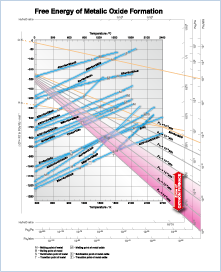
Ellingham diagram
- ●Visualization of atmosphere
- Conventional continuous furnaces would require experienced operators.
Especially at the time of starting up a furnace, operators need to properly check and validate such important operating factors like atmosphere purge,furnace temperature, and operation of each instruments.
KYK's unique Visualized Operation monitor panel can clearly and easily display real-time atmosphere of the furnace, which was only possible by the experienced operators before.
This new technology enables even inexperienced operators to properly operate and manage equipment at foreign production facilities.
Recommended equipment: Oxynon®Furnaces of all kinds. -